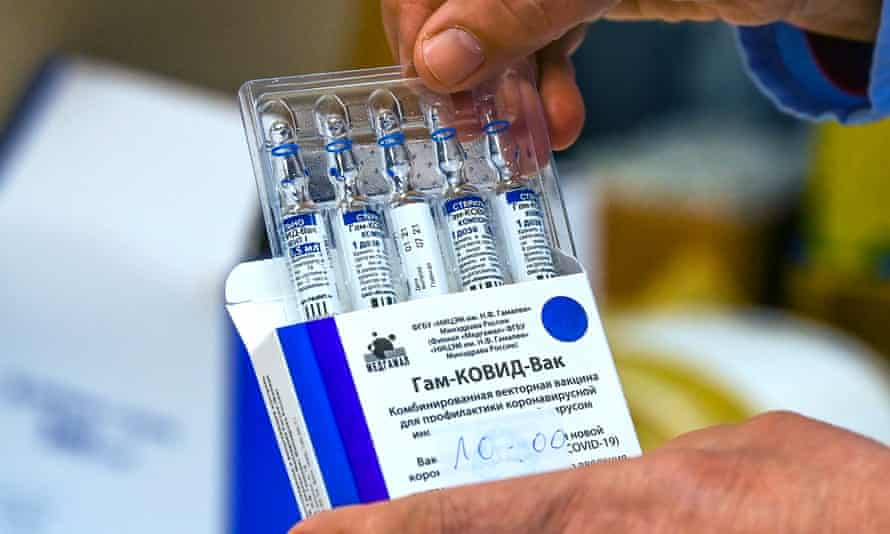
The Ghana Health Service (GHS) is beginning Phase 3 clinical trial of Sanofi and Sputnik Light COVID-19 vaccines in Kintampo, Navrongo and Dodowa in July 2021.
This follows the selection of Ghana as one of the countries for the Phase 3 clinical trial of the two COVID-19 vaccines from France and Russia respectively.
The Kintampo Health Research Centre is handling the clinical trial in the Kintampo area.
A total of 1500 volunteers have since been recruited for the trial in Kintampo for instance, the Director of the Kintampo Health Research Centre, Dr Kwaku Poku Asante has disclosed.
With Sputnik V, the first Russian made COVID-19 vaccine, which is already in use, a second dose is administered after three to four weeks for maximum protection but with Sputnik Light, it is just a single dose same as the Johnson & Johnson one.
It is developed by the Russian Gamaleya Research Institute of Epidemiology and Microbiology and it actually consists of the first dose of the Sputnik V vaccine, which is based on the Ad26 vector, and it can be stored at a normal refrigerator temperature of 2–8 °C (36–46 °F).
The Russian institute says this version, with an effectiveness of 79.4 percent, would be ideally suited for areas with acute outbreaks, allowing more people to be vaccinated quickly.
The Sputnik V vaccine works in a similar way to others developed by Oxford/AstraZeneca and Janssen/Johnson & Johnson. It uses a cold-type virus, engineered to be harmless, as a carrier to deliver a small fragment of the coronavirus to the body.
The other one, Sanofi, is by Sanofi S.A., which is a French multinational pharmaceutical company headquartered in Paris, France.
Sanofi is working with its development partner, GlaxoSmithKline, which is providing an adjuvant used in the vaccine. The companies have contracts with multiple countries.
Monitoring
The GHS Deputy Director, Public Health, Dr Franklin Asiedu-Bekoe has explained that the research division of the GHS will monitor efficacy of the vaccines by comparing those who will take the vaccine to those who will not.
Phase one and Phase two of the trials have already been cleared and it has now gotten to the phase three level, he said.
The trial of Sputnik Light will take about three months to complete while that of Sanofi will take about a year to complete, Dr Asiedu-Bekoe added.
Those who have already taken any of the COVID-19 vaccines don’t qualify for the trial same as pregnant women and under 18-year-olds.
Those who will be part of the trials will have to sign a consent form.
Kintampo and Malaria vaccine achievement
The Kintampo Health Research Centre in collaboration with GlaxoSmithKline (GSK) recently successfully worked on a malaria vaccine which received a commendation from the World Health Organisation (WHO). The malaria vaccine is currently being administered to children.
The Director of the Kintampo Health Research Centre, Dr Kwaku Poku Asante in a television interview with UTV said there was a discussion last Tuesday [July 22, 2021] on how the trial was going to be done.